The Joint Annual Scientific Meetings of the Endocrine Society of Australia and the Society for Reproductive Biology 2018
Experience with sodium–glucose co-transporter 2 inhibitors initiation in a public hospital diabetes outpatient population (#252)
Sodium–glucose co-transporter 2 (SGLT2) inhibitors are new group of medications which reduce glucose reabsorption in the proximal tubule leading to decrease in plasma glucose.1 In addition to glycaemic control, SGLT2 inhibitors improve the body weight and blood pressure (BP).2,3
Although SGLT2 inhibitors are well tolerated, they are associated with an increased risk of lower urinary tract and genital infections, as well as dehydration, urinary frequency, diabetic ketoacidosis, and bone fractures. 4,5,6,7
This is a retrospective descriptive study assessing the safety and efficacy measures of SGLT2 inhibitors in a real -world public diabetes outpatient setting.
Method
This is a retrospective study analyzing data from 50 patients with type 2 diabetes (T2DM) who were treated with dapagliflozin or empagliflozin in the diabetic clinics across Eastern Health, between October 2014 and February 2018.
All statistical computations were performed using SPSS version 23.0. Descriptive statistics were analysed followed by T tests, Pearsons and ANOVA correlations and Multivariate analysis.
Results
The mean age of the participants was 57.24 years, 52% were women and 60% of patients received Dapagliflozin. The SGLT2 inhibitor was stopped in 34% of the patients due to genital and urinary tract infection, worsening of glycaemic control, weight gain or polyuria (Table.1). No significant change noted in renal function.
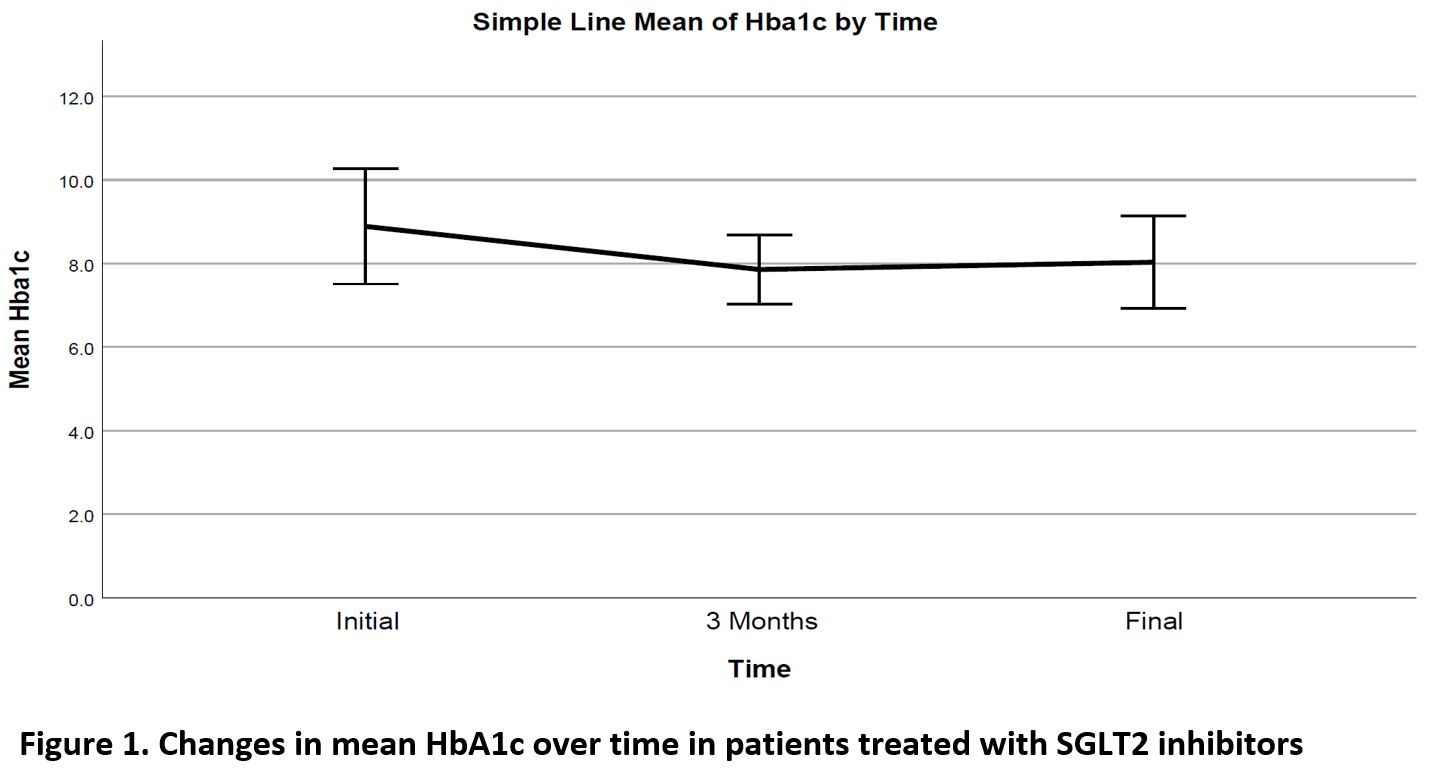
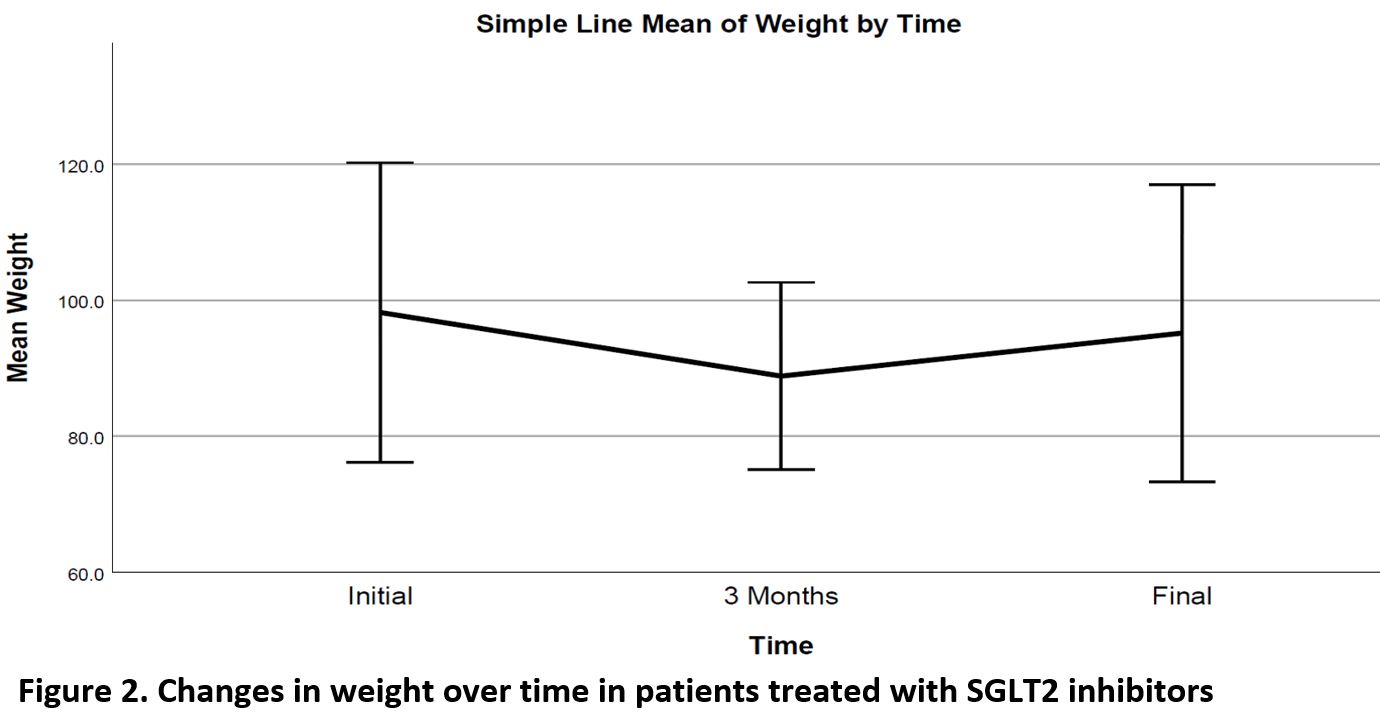
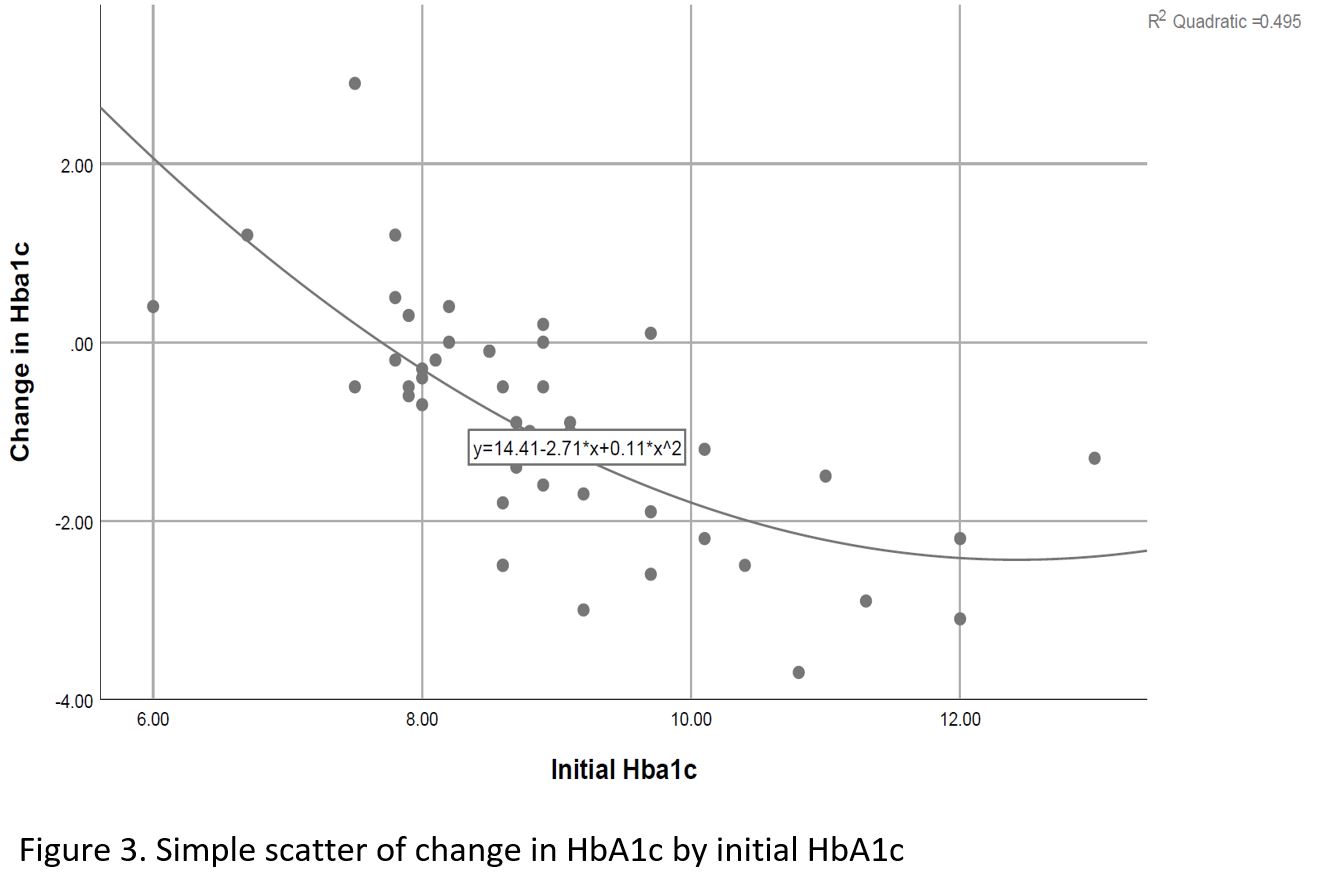
Significant decrease noted in Hemoglobin A1c (HbA1c), weight and diastolic BP (DBL), ALT and GGT over time, as well as a significant increase in HDL cholesterol level (Table 2, Figures 1,2). Change in HbA1c was related to initial HbA1c and independent of age, weight or initial renal function (Figure 3).
Discussion
The efficacy parameters of SGLT2 inhibitors have again been shown in this study. Despite the significant benefits in glycaemic control, weight, blood pressure and HDL cholesterol levels, the SGLT2 inhibitor was discontinued in nearly 1 out of 3 patients, mainly due to side effects.
- Andre ´ J. Scheen. Pharmacodynamics, Efficacy and Safety of Sodium–Glucose Co-Transporter Type 2 (SGLT2) Inhibitors for the Treatment of Type 2 Diabetes Mellitus. Drugs. 2015;75:33–59.
- Tikkanen I, Chilton R, et al. Potential role of sodium glucose cotransporter 2 inhibitors in the treatment of hypertension. Curr Opin Nephrol Hypertens. 2016;25:81–6.
- Zinman B, Wanner C, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N ENGL J MED. 2015;373:2117-28.
- Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335-80.
- Bailey CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci. 2011;32(2):63–71.
- Palmer BF, Clegg DJ, et al. Diabetic ketoacidosis, sodium glucose transporter-2 inhibitors and the kidney. J Diabetes Complications. 2016;30(6):1162-6.
- Johnsson KM, Ptaszynska, et al. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27(5):479-84.